

Patients with malignant tumors, other serious systemic diseases and psychosis.Those who are not pregnant, breastfeeding and pregnant but who do not take effective contraceptive measures.Advancing of focus in Pulmonary imaging to >50% in 24-48 hours.Arterial oxygen partial pressure (PaO2)/oxygen absorption concentration (FiO2) ≤ 300MMHG.Confirmed Pneumonia with chest radiography and computer tomography.Confirmed 2019-nCoV infection with RT-PCR Laboratory test.CD4 + T, CD4 + T killer cells Granulocyte macrophage colony factor BLC-2 VEGF-R angiopoetin-1, angiopoetin-2 Total antioxidant capacity (TAC) Total oxidant capacity (TOC). Proinflammatory (IL1-β, IL-6, TNFα, INF-γ) and anti-inflammatory (IL-2, IL-4, IL-10, IL-13) cytokines will be examined in venous blood in order to determine the immune modulatory effect of stem cells. immunoassay kits will be used for analyses in accordance with the manufacturer's instructions.īiochemical parameters of the liver, such as alanine transaminase (ALT), aspartate transaminase (AST), total protein, albumin, total bilirubin, direct bilirubin, and alkaline phosphatase (ALP) levels, will be measured in the venous blood samples. Investigators also analyzed the caspase-3 system in the blood. The blood will be analyzed for the expression levels of growth factors, including vascular endothelial growth factor, fibroblast growth factor, platelet derived-growth factor, epidermal growth factor, transforming growth factor beta, hepatocyte growth factor, nerve growth factor, VEGF receptor (VEGFR), angiopoietin1 (Angpt-1), and Angpt-2, using sandwich enzyme-linked immune sorbent assays (ELISAs). Application: 3 million cells / kg IV - 6.Application: 3 million cells / kg IV - 3.

Application: 3 million cells / kg IV - 1 day.Mesenchymal stem cells originating from allogenic umbilical cord produced under GMP conditions will be administered in 3 times, with doses indicated below, intravenously within 1 week.

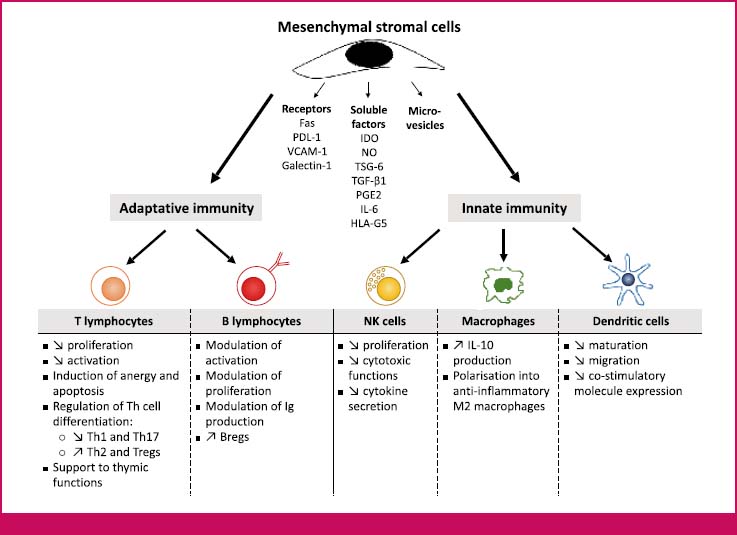
Group 1: patients that are not on a ventilator (n=10) Group 2: patients that are on a ventilator and will receive saline injections (n=10) Group 3: patients that are on a ventilator and will receive MSC transplantation injections (n=10) Patients diagnosed with COVID-19 infection as clinically, radiologically and laboratory-wise will be divided into three groups: It is to accelerate the recovery in organ damage by increasing growth factors by means of MSCs.Correcting immunosuppression in patients and increasing the fight against COVID-19 virus by CD4+T, CD+8T cellular cell arrangement,.To provide immune modulation to patients with COVID-19 who are taken to intensive care and resistant to treatment by performing MSCs transplantation and to reduce the damage caused by cytokin storm to tissues and organs,.This was attributed towards attenuation of pro-inflammatory cytokine secretion, inflammatory cell recruitment and increased alveolar macrophages content. MSCs application proved therapeutic efficiency during influenza infection resulting in reduced impairment of alveolar fluid clearance and lung injury. Mesenchymal stem cells (MKH) not only inhibit the abnormal activation of T lymphocytes and macrophages, but also encourage them to differentiate into regulatory T cell subsets (Treg) clusters and anti-inflammatory macrophages. When the virus invades the body, dentric cells can activate macrophages, lymphocytes and natural killer cells. This condition is associated with cytokine storm in the body. In severe patients, ARDS and multiple organ failure can be seen. The COVID-19 corona virus epidemic has spread from Wuhan, China to the whole world, and has been declared a pandemic by WHO worldwide.


 0 kommentar(er)
0 kommentar(er)
